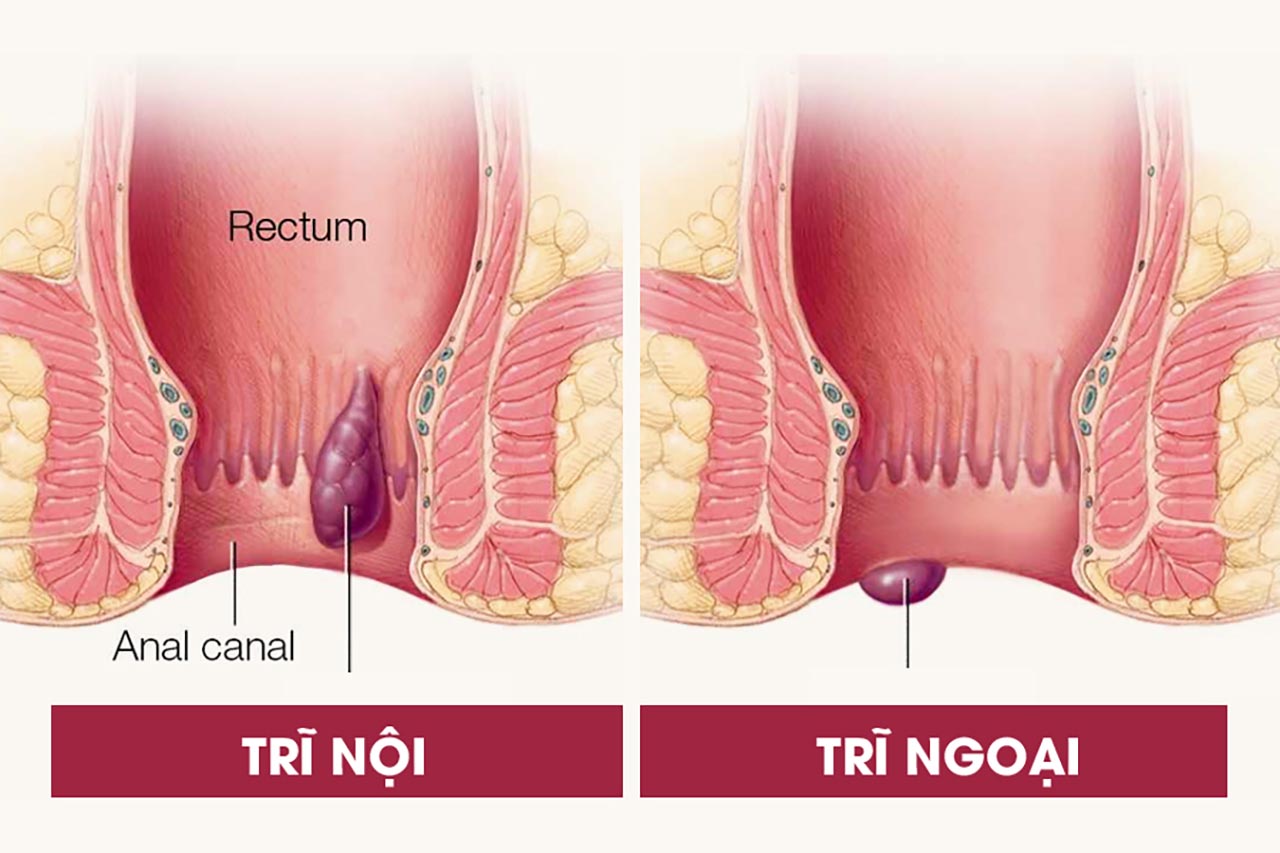
The shell mentioned here is the capsule. Capsules or capsules are pills made up of a hard or soft shell containing drugs in them and are usually used for oral administration. The main component of the capsule shell is gelatin.

The Drug Administration (Ministry of Health) has requested health departments to check the quality of gelatin – Picture: N.C.T.
Gelatin is found in connective tissue, bones, and skin of animals and is a product produced from pig and cow skin. From the skin of pigs and cows, collagen is partially hydrolyzed to turn into gelatin. Thus, in order to make a drug that is a capsule, it must have a capsule shell made from gelatin as a raw material. And gelatin used in the pharmaceutical industry to produce drugs must be pharmaceutical gelatin.
Strict origin.
Everything for drug production such as pharmaceutical ingredients, excipients… must meet pharmaceutical standards. As for gelatin, it must first be determined whether it is derived from pig skin or cow skin, because some people do not use products derived from pigs. Capsule shells made from gelatin from pigs are not accepted as medicine in Muslim countries (they only use capsule shells made from cow skin, there was a case where a big pharmaceutical company had a hard time apologizing for mistakenly asking for help). The drug is allowed to circulate in Indonesia with capsule shell made from pig skin!).
“Standard for heavy metal testing for medicinal materials, people almost only pay attention to lead and not chromium”
For many advanced countries, gelatin from bovine hides used for medicinal purposes must be certified as being produced from cows that do not have BSE. The standards of gelatin for medicinal use are specified in the pharmacopoeias and must be strictly adhered to. Besides physical and chemical standards (such as pharmaceutical gelatin has a freezing point, that is, heating gelatin dissolved in water when cooled, it will solidify – like frozen meat dishes in the North of our country are frozen solids). thanks to gelatin), there are also standards to determine that it does not contain bacteria, does not contain toxic substances above the allowable limit (such as no heavy metals, typically lead).
Capsule is the most commonly used drug (along with tablets), so the testing of pharmacological standards of ingredients, including gelatin, is very strict to avoid harm to drug users.
… Still “passing”?
Recently, there was a scandal of pills containing carcinogens containing carcinogens in China, which shocked public opinion. Why so? This is because the companies that produce the capsule shells in their country for a profit have used waste products from leather shoemaking to extract the gelatin used to make capsule shells. If you take raw materials from leather that has undergone tanning processing with industrial chemicals, it is important to avoid the fact that the product does not contain toxic substances. So during the inspection, nine famous Chinese pharmaceutical companies were ordered by the government to publish in the media a list of drugs contaminated with chromium metal.
Chrome is a heavy metal (like lead, mercury), chemical symbol Cr, usually present in the form of compounds such as chromate… Cr has very little role in life, if present. present above the allowable level in the body will cause toxicity. Cr will cause acute toxicity when present in living organisms at concentrations from 3.3-15mg/kg. According to Chinese regulations, the allowed Cr content is not more than 2mg per kg of pharmaceutical products. It has long been known that Cr causes cancer due to changes in the DNA structure of chromosomes.
Due to the scandal just mentioned, medical experts were startled because they did not pay enough attention to the production of gelatin used as medicine. No one expected because of illicit profits that people can destroy their consciences to produce gelatin from leather waste. And the standard of heavy metal testing for medicinal materials, people almost only pay attention to lead and don’t care about chromium. For this reason, the Drug Administration of Vietnam (Ministry of Health) has just sent a document to the health departments of the provinces and cities, and drug capsule manufacturers requesting review, inspection to determine the origin of raw materials and quality control. gelatin, gelatin capsule shell, added chromium test criteria in gelatin and capsule shell.