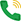That’s the information given at the workshop to introduce good manufacturing practices (GMP) for health supplements, organized by the Food Safety Authority-Ministry of Health. held on March 29. Attending the seminar were representatives of 52 enterprises producing and trading industrial foods in the North and Central regions.
Director of Food Safety Department Nguyen Thanh Phong assessed that foodstuffs are a very hot field at the moment and need to be managed more tightly so that businesses that do not qualify for production facilities but deliberately bring products to market. not guaranteed. Meanwhile, dietary supplements are a strength of Vietnam when we have the second largest traditional medicine in the world. Despite having the advantage of huge source of medicinal herbs, in order to develop it, it is necessary to apply scientific and technical advances. If the product is many but the quality is not good, we will lose at home. Therefore, the view of the Ministry of Health is to create conditions for enterprises to develop in accordance with the law, promote the strengths of traditional medicine in the country, and encourage enterprises to promote export-oriented development.
Also according to Mr. Nguyen Thanh Phong, the Ministry of Health advocates to have a roadmap to apply Good Manufacturing Practices (GMP) to dietary supplements from mid-2017. According to the draft document guiding good manufacturing practices ( GMP) Health supplement consists of 10 main contents with 5 items: quality management system, personnel and training, factory and equipment, hygiene, documentation. In particular, in terms of workshops and equipment, the principle for a food factory to meet GPM standards is to be far from sources of pollution, insects and harmful animals; have a separate area for storage, distribution, processing, packaging and testing; have a suitable ventilation system; well maintained, well maintained. In particular, the production area must be arranged according to the one-way principle, ensuring the appropriate parameters, easy cleaning, infection control, the storage area must be arranged separately, the testing area must be separate. must be separated from the production area… After this document is issued, the Ministry of Health will issue Decrees and circulars for implementation.
And according to Mr. Nguyen Thanh Phong, the view of the Food Safety Department is that the application of GMP should not be too sudden. Currently, there are many health supplement enterprises that have not been able to respond quickly to GMP, so the Department will study a roadmap so as not to suffocate businesses but must aim to create conditions for enterprises to switch to GMP application. On that basis, the mandatory deadline for applying GMP industrial foods may be a little later because according to ASEAN recommendations, it is only mandatory from 2021. With Vietnam’s conditions, we can apply it earlier but still must take into account the specific conditions of the business community.
The head of the Department of Food Safety also said that he will study a preferential mechanism for enterprises applying GMP foodstuffs. Currently, we have an incentive that the validity period of product announcement at GMP-certified manufacturing plants (or equivalent or higher) is 5 years instead of 3 years like products in other countries. the factory has not met this standard.