Anh Van Thi Pham1,#, Anh Quang Luong2,3,#, Dung Kim Thi Dao4, Vy Nhat Dao Nguyen4, Tam
Cong Nguyen4, Thoa Thi Dao4, Long Hai Luu5,6, Lan Hai Luu5,6, Gioi Huy Dong6, Huong Thu Thi
Bui6, Tung Thanh Tran1, Duong Thuy Dau1, Hai Van Nguyen7, Minh Hai Luu5,# and Loan Thanh
Thi Nguyen1,*
1Department of Pharmacology, Hanoi Medical University, Hanoi 10000, Vietnam
2Department of Pharmacy and Medical Equipment, National Burn Hospital, Hanoi 10000, Vietnam
3Vietnam Military Medical University, Hanoi 10000, Vietnam
4DKD International Production Joint Stock Company, Ho Chi Minh 70000, Vietnam
5Nhat Hai New Technology Joint Stock Company, Hanoi 10000, Vietnam
6Vietnam National University of Agriculture, Hanoi, Vietnam
7Hanoi University of Pharmacy, Hanoi, Vietnam
Abstract:
Background: Burn injuries and skin ulcers are important health problems resulting in physical and psychological
scars and chronic disabilities. This study investigated the wound-healing effects of liposomal nanocurcumin and PL
pro nanocurcumin on thermal burns in rats and doxorubicin-induced skin ulcers in mice and their systemic toxicity.
Methods: Having subjected to a cylindrical hot steel rod onto the dorsum, burned lesions were covered topically with
silver sulfadiazine/liposomal nanocurcumin/PL pro nanocurcumin twice a day for 21 days. Besides, the other skin
lesions which were induced by a single intradermal injection of doxorubicin on the dorsal region were topically
administered with dimethyl sulfoxide/liposomal nanocurcumin/PL pro nanocurcumin twice a day for 21 days.
Results: The results indicated that liposomal nanocurcumin and PL pro nanocurcumin significantly reduced the
wound size, increased the hydroxyproline content in animals’ skin, and improved the histopathological structure of
the affected tissues. Specifically, liposomal nanocurcumin demonstrated better healing results than PL pro
nanocurcumin on thermal burns. Furthermore, topical administration of liposomal and PL pro nanocurcumin was
deemed not to exert any systemic toxicity to the wounded animals by not influencing considerably the hematological
parameters and renal and hepatic functions and altering the histology of the liver and kidney. Additionally, liposomal
nanocurcumin and PL pro nanocurcumin with average sizes of 206 nm and 344 nm were well-dispersed in water,
accentuating that the disadvantages of limited water solubility have been overcome.
Conclusion: Thus, liposomal nanocurcumin and PL pro nanocurcumin exerted effective effects on burned wounds
and skin ulcers whilst triggering no systemic toxicity in wounded animals.
Keywords: Liposomal nanocurcumin, PL pro nanocurcumin, Burn, Skin ulcer, Healing, Wounded animals
1. INTRODUCTION
Curcumin has perennially been referred to as a
bioactive, important compound found in nature. To be
specific, this substance is isolated from Curcuma longa L.,
which belongs to the Zingiberaceae species with a
scientific name of (1e,6e)-1,7-bis(4-hydroxy-3’-methoxy
phenyl)-1,6-heptadiene-3,5-dione [1, 2]. For years,
curcumin has been profoundly embedded in the sociocultural lifestyle of different peoples, especially Asians. It
is not only considered a nature-based colorant, flavouring
agent, and food preservative in many local cuisines but is
also utilized for the sake of curing many illnesses.
Regarding the pharmacological properties, many
beneficial biological features of curcumin have been
identified, such as anti-oxidant, anti-inflammatory, antimicrobial, anti-mutagenic, anti-tumoral, anti-angiogenesis activities, and wound healing effects [3-10].
Besides the evidence for the diversity of bioactivities of
curcumin, this substance barely exhibits any toxicity at
high doses when used for clinical treatment purposes
[11-16]. Therefore, this statement does pave the way for
curcumin to be popularly studied in various research
studies relating to the dysregulation of many human
organs. In particular, when it comes to some skin
disorders and damages with many free radicals emitted,
curcumin improves the skin with its capability to eradicate
reactive oxygen species and attenuates local inflammation
by inhibiting the nuclear receptor NF-κB [17].
Furthermore, treating skin disorders with curcumin does
help to shorten the wound healing time, enhance the
deposition of collagen, and also increase the density of
fibroblasts and vasculatures, thus reinforcing the healing
of the affected tissue with different levels of severity
[18-20].
Notwithstanding those mentioned beneficial features,
curcumin does exhibit many limitations, including poor
water solubility and physicochemical instability, less
bioactive absorption, rapid metabolization, and low
penetration and targeting efficacy [21-24]. Meanwhile,
nanoformulation has been testified regarding the potential
of targeted delivery to the tissue of interest that leads to
enhanced bioavailability and bioactivity and better drug
carriage [25-28]. With the intention of taking advantage of
this compound and simultaneously solving its drawbacks,
the advent of nanocurcumin has shown to be prominent.
There are currently several methods to encapsulate
curcumin molecules at the nanoscale, and each uses a
different but suitable nanocarrier [29].
Burn injuries and skin ulcers are still considered
important health problems affecting both genders and all
age groups, resulting in physical and psychological scars
and leading to chronic disabilities [30-33]. To date,
research on burns has generated sustained interest over
the past few decades. In current burn therapy, silver
sulfadiazine has been presented as the gold standard in
topical second-degree burn treatment because of its
antibacterial activities [34, 35]. However, the effect of
silver sulfadiazine stems from the toxicity towards
keratinocytes and fibroblasts, hence decelerating the
1. INTRODUCTION
Curcumin has perennially been referred to as a
bioactive, important compound found in nature. To be
specific, this substance is isolated from Curcuma longa L.,
which belongs to the Zingiberaceae species with a
scientific name of (1e,6e)-1,7-bis(4-hydroxy-3’-methoxy
phenyl)-1,6-heptadiene-3,5-dione [1, 2]. For years,
curcumin has been profoundly embedded in the sociocultural lifestyle of different peoples, especially Asians. It
is not only considered a nature-based colorant, flavouring
agent, and food preservative in many local cuisines but is
also utilized for the sake of curing many illnesses.
Regarding the pharmacological properties, many
beneficial biological features of curcumin have been
identified, such as anti-oxidant, anti-inflammatory, antimicrobial, anti-mutagenic, anti-tumoral, anti-angiogenesis activities, and wound healing effects [3-10].
Besides the evidence for the diversity of bioactivities of
curcumin, this substance barely exhibits any toxicity at
high doses when used for clinical treatment purposes
[11-16]. Therefore, this statement does pave the way for
curcumin to be popularly studied in various research
studies relating to the dysregulation of many human
organs. In particular, when it comes to some skin
disorders and damages with many free radicals emitted,
curcumin improves the skin with its capability to eradicate
reactive oxygen species and attenuates local inflammation
by inhibiting the nuclear receptor NF-κB [17].
Furthermore, treating skin disorders with curcumin does
help to shorten the wound healing time, enhance the
deposition of collagen, and also increase the density of
fibroblasts and vasculatures, thus reinforcing the healing
of the affected tissue with different levels of severity
[18-20].
Notwithstanding those mentioned beneficial features,
curcumin does exhibit many limitations, including poor
water solubility and physicochemical instability, less
bioactive absorption, rapid metabolization, and low
penetration and targeting efficacy [21-24]. Meanwhile,
nanoformulation has been testified regarding the potential
of targeted delivery to the tissue of interest that leads to
enhanced bioavailability and bioactivity and better drug
carriage [25-28]. With the intention of taking advantage of
this compound and simultaneously solving its drawbacks,
the advent of nanocurcumin has shown to be prominent.
There are currently several methods to encapsulate
curcumin molecules at the nanoscale, and each uses a
different but suitable nanocarrier [29].
Burn injuries and skin ulcers are still considered
important health problems affecting both genders and all
age groups, resulting in physical and psychological scars
and leading to chronic disabilities [30-33]. To date,
research on burns has generated sustained interest over
the past few decades. In current burn therapy, silver
sulfadiazine has been presented as the gold standard in
topical second-degree burn treatment because of its
antibacterial activities [34, 35]. However, the effect of
silver sulfadiazine stems from the toxicity towards
keratinocytes and fibroblasts, hence decelerating the
wound healing process and probably triggering serious
cytotoxic effects on the host cells. Furthermore, there are
quite a number of articles to be reviewed on some
emerging sliver-sulfadiazine-resistant organisms [36-38].
Additionally, in the treatment of skin ulceration, dimethyl
sulfoxide (DMSO) is perennially one of the most proposed
remedies since it can easily infiltrate into the affected area
and scavenge free radicals, which is an important etiology
of serious tissue damage [39]. However, for the time
being, the accessibility of DMSO and other effective
agents for skin ulcers is still restricted. Therefore, seeking
a safer and more effective treatment approach towards
skin lesions has been critically demanded in healthcare
practice, particularly those caused by thermal or chemical
triggers.
The beneficial effects and potentials of curcumin in
different nano-based dosage forms, including liposomal
nanocurcumin and PL pro nanocurcumin, with the
assessment in terms of healing effects in both burned and
ulcerated skin lesions, as well as the systemic toxicity in
the experimental model remain unclear. In the present
study, we investigated the wound-healing effect of
liposomal nanocurcumin and PL pro nanocurcumin on
thermal burns in rats and doxorubicin-induced skin ulcers
in mice and their systemic toxicity in ulcerated
experimental animals.
2. MATERIALS AND METHODS
2.1. Preparation of Liposomal Nanocurcumin and PL
Pro Nanocurcumin Formula
2.1.1. Liposomal Nanocurcumin
Firstly, nanocurcumin was created. The dispersed
phase by dissolving curcumin in ethanol was prepared
with a volume ratio of 4/5. Then, a carrier mixture
consisting of polyethylene glycol (PEG) and ethylene glycol
by dispersing polyethylene glycol and ethylene glycol well
in water was made with a ratio of approximately 1.5/6/2
for polyethylene glycol/ethylene glycol /water, under
ultrasonic vibration for about 2 hours at room
temperature. A homogeneous mixture was made by mixing
the dispersed phase/liquid in the previous step, the carrier
mixture, and the emulsifier lecithin such that the ratio of
curcumin/PEG/lecithin in this homogenizer was 1.6/1.5/2
using an emulsifier. Nano-emulsions of curcumin were
created by allowing the mixture to homogenize overnight
and then centrifugated at room temperature at 5000 rpm
for about 10 minutes, which was repeated six times. After
obtaining curcumin nano-emulsions, nano-curcumin and
phospholipids were weighed and prepared according to
the respective ratio of 1/1. Liposomal nanocurcumin was
obtained by putting the prepared mixture into the
emulsifier and heating it at 120oC within 4 hours.
2.1.2. PL Pro Nanocurcumin
Nano curcumin was prepared using the method
described above. PL pro included 18% phosphatidylcholine, 21% cholesterol, 27% lecithin, 9.5% folic acid,
15% nano curcumin, 3% tocopherol, 3% xanthan gum, 3%
wound healing process and probably triggering serious
cytotoxic effects on the host cells. Furthermore, there are
quite a number of articles to be reviewed on some
emerging sliver-sulfadiazine-resistant organisms [36-38].
Additionally, in the treatment of skin ulceration, dimethyl
sulfoxide (DMSO) is perennially one of the most proposed
remedies since it can easily infiltrate into the affected area
and scavenge free radicals, which is an important etiology
of serious tissue damage [39]. However, for the time
being, the accessibility of DMSO and other effective
agents for skin ulcers is still restricted. Therefore, seeking
a safer and more effective treatment approach towards
skin lesions has been critically demanded in healthcare
practice, particularly those caused by thermal or chemical
triggers.
The beneficial effects and potentials of curcumin in
different nano-based dosage forms, including liposomal
nanocurcumin and PL pro nanocurcumin, with the
assessment in terms of healing effects in both burned and
ulcerated skin lesions, as well as the systemic toxicity in
the experimental model remain unclear. In the present
study, we investigated the wound-healing effect of
liposomal nanocurcumin and PL pro nanocurcumin on
thermal burns in rats and doxorubicin-induced skin ulcers
in mice and their systemic toxicity in ulcerated
experimental animals.
2. MATERIALS AND METHODS
2.1. Preparation of Liposomal Nanocurcumin and PL
Pro Nanocurcumin Formula
2.1.1. Liposomal Nanocurcumin
Firstly, nanocurcumin was created. The dispersed
phase by dissolving curcumin in ethanol was prepared
with a volume ratio of 4/5. Then, a carrier mixture
consisting of polyethylene glycol (PEG) and ethylene glycol
by dispersing polyethylene glycol and ethylene glycol well
in water was made with a ratio of approximately 1.5/6/2
for polyethylene glycol/ethylene glycol /water, under
ultrasonic vibration for about 2 hours at room
temperature. A homogeneous mixture was made by mixing
the dispersed phase/liquid in the previous step, the carrier
mixture, and the emulsifier lecithin such that the ratio of
curcumin/PEG/lecithin in this homogenizer was 1.6/1.5/2
using an emulsifier. Nano-emulsions of curcumin were
created by allowing the mixture to homogenize overnight
and then centrifugated at room temperature at 5000 rpm
for about 10 minutes, which was repeated six times. After
obtaining curcumin nano-emulsions, nano-curcumin and
phospholipids were weighed and prepared according to
the respective ratio of 1/1. Liposomal nanocurcumin was
obtained by putting the prepared mixture into the
emulsifier and heating it at 120oC within 4 hours.
2.1.2. PL Pro Nanocurcumin
Nano curcumin was prepared using the method
described above. PL pro included 18% phosphatidylcholine, 21% cholesterol, 27% lecithin, 9.5% folic acid,
15% nano curcumin, 3% tocopherol, 3% xanthan gum, 3%
Camellia sinensis extract, and 0.5% Aloe vera extract, then
nano curcumin and PL pro were mixed according to the
corresponding volume ratio of 1/1 in the emulsifier. After
two hours, PL pro nanocurcumin was obtained.
2.2. Particle Size
Liposomal nanocurcumin and PL pro nanocurcumin
samples were tested to determine particle size. This
process was carried out utilising Malvern Mastersizer
(Malvern Instruments Ltd., United Kingdom). The
measurement was carried out by dissolving particles in
water before measuring. The system temperature was
kept at about 25°C. Hence, the solution was checked in
terms of limitations regarding solubility.
The stability of liposomal nanocurcumin and PL pro
nanocurcumin was determined through an accelerated
aging test. This test simulates the aging process over time
by subjecting the samples to high temperatures to
artificially expedite the aging process. The accelerated
aging process was conducted using an incubator (Daihan
Scientific, South Korea), maintaining a constant
temperature of 40°C for six months.
2.3. Experimental Animals
Male and female Wistar rats weighing 180 ± 20g and
seven-week-old male and female Swiss albino mice were
obtained from the National Institute of Hygiene and
Epidemiology, Hanoi, Vietnam. All experimental protocols
were in accordance with the National Guideline (reference
number: 141/QD-K2DT). This study was approved by the
Scientific Board Committee of Hanoi Medical University,
Vietnam (ref number: IRB00003121). All animals were
housed in a controlled environment (25 ± 1ºC under 65 ±
5% humidity and a 12-hour light and dark cycle) with ad
libitum to access the standard rodent diet and water. The
animals were given for at least one week to acclimate
before starting the experiments.
2.4. Healing Effect of Topical Administration of
Liposomal Nanocurcumin and PL Pro Nanocurcumin
Creams
2.4.1. Thermal Burn in Rats
We followed the previously reported model of thermal
burns on rats. A total of 50 rats were randomly divided
into five groups of ten animals. The rats were anesthetized
with a single intraperitoneal injection of 250 mg/kg
chloralhydrate (Sigma Aldrich, St. Louis, MO, USA). As
preparation, they were shaven at the dorsum with an
electric shaver and later sterilized with 70% alcohol. All
animals, except the normal control group, were subjected
to thermal burns on the back of each rat by using a
standard burning technique [40]. Burn wounds were
formed by applying a 200-gram cylindrical stainless-steel
rod (2.5 cm diameter) without any pressure, which was
pre-heated to 100°C in boiling water with the thermal
equilibrium confirmed by a monitoring thermometer, onto
the shaven skin for 35 seconds. All animals were
resuscitated immediately with Lactated Ringer’s solution
(2 ml/100 g body weight) intraperitoneally. Following the
burning, each animal was placed in a separate cage, and
the affected areas were covered with 0.3 g silver
sulfadiazine, liposomal nanocurcumin, or PL pro
nanocurcumin twice a day for 21 days. The vehicle-treated
burned rats topically received sterile distilled water (Fig.
1A).
2.4.2. Doxorubicin-induced Skin Ulcer in Mice
Fifty mice were randomly divided into five groups of
ten animals. Mice were anesthetized with an
intraperitoneal injection of 350 mg/kg chloralhydrate.
After anesthesia, the dorsal regions were shaven with an
electric shaver and sterilized with 70% alcohol. All
animals, except the normal control group, were induced
skin ulcers by a single intradermal injection of 0.2 ml
doxorubicin 1 mg/0.5 ml (Doxorubicin Ebewe, Austria)
[41]. Then, each animal was placed in a separate cage.
Seven days after the injection of doxorubicin, the vehicletreated ulcerated mice topically received sterile distilled
water. The other ulcerated mice were topically applied 0.3
ml DMSO (Sigma Aldrich, St. Louis, MO, USA) twice a day,
0.3 g liposomal nanocurcumin or PL pro nanocurcumin
twice a day for 21 days (Fig. 1B).
2.4.3. Measurement of the Wound Size
Wound sizes of animals in two experiments were
measured using a digital camera with one camera lens and
from a constant focal distance. The area of the wound was
measured in a blind manner using ImageJ basics software
ver 1.38, which was recognized as software for measuring
the area in medical experimental research by the World
Health Organization.
2.4.4. Determination of the Hydroxyproline Content
At the end of two experiments, mice and rats were
anesthetized with chloralhydrate, and skin samples were
collected from each animal. The concentration of
hydroxyproline in the skin was evaluated according to the
Stegemann H. and Stalder K method [42]. Briefly, 20 to 30
mg of skin tissues were put into hydrolytic tubes with 2
mL HCl 6N. These tubes were incubated at 115°C. After
24 hours, the hydrolyzed fluid was collected into the test
tubes. Each test tube included 0.2 mL hydrolyzed fluid of
samples, 1.8 mL distilled water, and 1 mL chloramine T.
These test tubes were shaken and kept at room
temperature for 20 minutes. Then, 2 mL pechloric acid 4M
was added, shaken well, and let stand for 5 minutes at
room temperature. 4-Dimethylaminobenzaldehyde 10%
was added, shaken well, and kept in a bain-marie at 60oC
for 15 minutes. These tubes were cooled down to room
temperature and measured the light of 560 nm wavelength
absorption (Shimadzu, Japan).
2.4.5. Histopathological Evaluation
The ulcerated skin tissue samples were collected for
histopathological examinations. Histopathological evaluation was carried out randomly in 30% of each group.
These tissue samples were fixed in 10% neutral-buffered
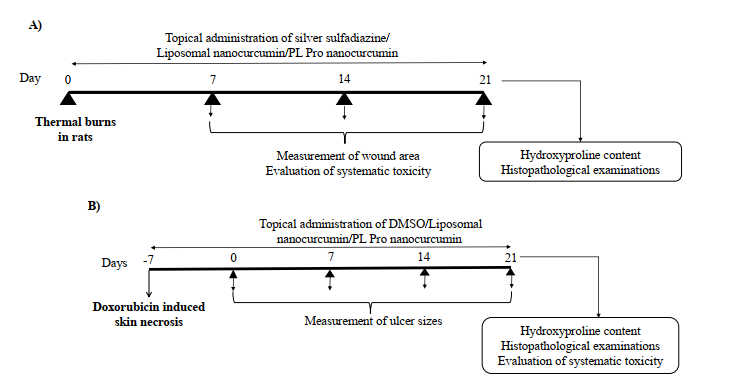
Fig. (1). Experimental protocols. (A) Experimental protocol for evaluating the effects of topical administration of liposomal nanocurcumin
and PL pro nanocurcumin creams on thermal burns in rats. (B) Experimental protocol for evaluating the effects of topical administration
of liposomal nanocurcumin and PL pro nanocurcumin creams on doxorubicin-induced skin necrosis in mice
formalin solution before they were embedded in paraffin
wax and cut into 5 μm-thick sections to be stained with
hematoxylin and eosin (H&E). The pathologist who
examined the slides was blind to group allocation. Under
histopathological examinations, inflammation, epithelization, neovascularization, and necrosis were evaluated.
2.5. Evaluation of systemic toxicity of topical
administration of liposomal nanocurcumin and PL
pro nanocurcumin creams in wounded animals
Blood samples were collected from each animal. The
systemic effects were quantified through general
conditions, including body weight changes in mice.
Moreover, the hematopoietic function was evaluated
through red blood cell count, hemoglobin, hematocrit,
total white blood cells, and platelet count. The liver
damage was examined through aspartate aminotransferase level (AST) and alanine aminotransferase level
(ALT), and the liver function was measured through total
bilirubin, albumin, and total cholesterol. Furthermore,
kidney function was examined through creatinine level.
Follow-up parameters were checked at the time points
before applying the products after 10 and 21 days in the
thermal burn model in rats (Fig. 1A). In the doxorubicininduced skin ulcer model in mice, blood samples were
obtained after 21 days of treatment (Fig. 1B).
At the end of the experiments, animals were
euthanized after blood collection, and the internal organs
(heart, liver, spleen, kidney, and lung) were removed and
observed for any gross lesions. The liver and kidneys of
30% of the animals in each group were preserved in a 10%
buffered formaldehyde solution for histopathological
studies using hematoxylin and eosin (H&E) staining by a
researcher blinded to the study.
2.6. Data Analysis
Sigmaplot 12.0 (SYSTA Software Inc, Richmond, CA,
USA) was used for statistical analysis. Obtained data were
expressed as the mean ± S.D and compared with either
one-way-ANOVA, followed by the post hoc StudentNewman-Keuls test for multiple comparisons or Fisher’s
Exact test for two proportions. Statistically significant
differences were considered when the p-value was less
than 0.05.
3. RESULTS
3.1. Particle Size
Liposomal nanocurcumin and PL pro nanocurcumin
with average sizes of 206 and 344 nm were well-dispersed
in water, indicating that the disadvantages of limited
water solubility have been overcome. Furthermore, the
results of the accelerated aging study revealed that after
six months of accelerated aging at 40°C, both liposomal
nanocurcumin and PL pro nanocurcumin remained stable
in particle size (Fig. S1).
3.2. Healing Effects of Liposomal Nanocurcumin and
PL Pro Nanocurcumin on Thermal Burns in Rats
3.2.1. Effect on the Wounded Area
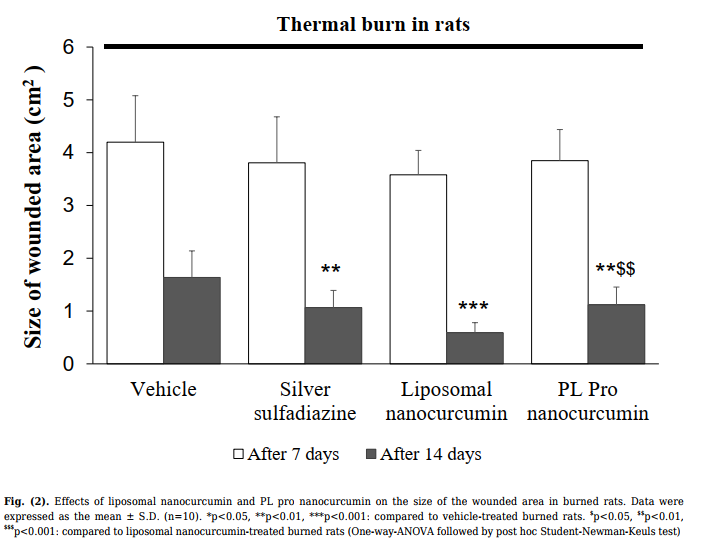
As shown in Fig. (2), after 7 days of treatment, there
was no difference in burned area between groups. After 14
days of administration, silver sulfadiazine, liposomal
nanocurcumin, and PL pro nanocurcumin markedly
reduced the wounded area compared to the vehicletreated model group (vehicle-treated burned group vs.
silver sulfadiazine-treated burned group, p=0.003; vehicletreated burned group vs. liposomal nanocurcumin-treated
group, p<0.001; vehicle-treated burned group vs. PL pro
nanocurcumin-treated group, p=0.003). The burned area
of liposomal nanocurcumin-treated rats significantly
decreased compared with the PL pro nanocurcumintreated rats (p=0.006).
After 21 days of treatment, the burn lesions of vehicletreated rats were not completely healed. The rate of burn
wound healing in the silver sulfadiazine- and liposomal
nanocurcumin-treated groups was 50%, with a statistically
significant difference compared to the vehicle-treated
group (p=0,033; Fisher’s exact test). PL pro nanocurcumin-treated rats had a wound healing rate of 20%.
There was no markedly significant difference in the rate of
wound healing between the vehicle-treated group and the
PL pro nanocurcumin-treated group (p>0.05).
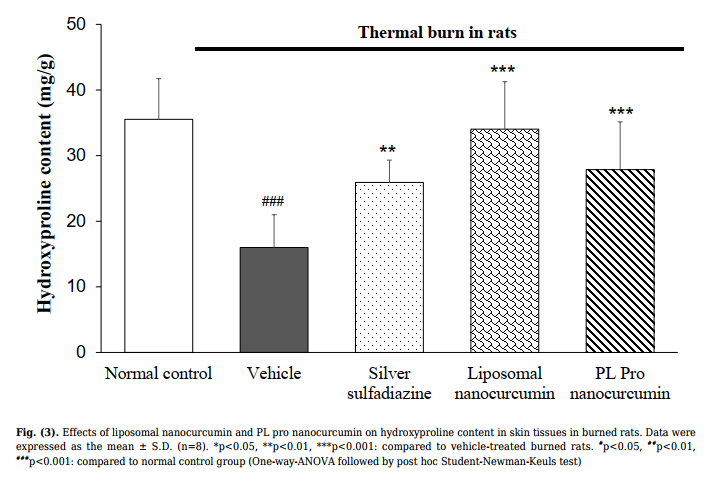
3.2.2. Effect on Hydroxyproline Content
As shown in Fig. (3), the content of hydroxyproline in
the rat skin sample of the vehicle-treated group was
significantly lower than the normal control group
(p<0.001). Compared with the vehicle-treated model
group, treatment of silver sulfadiazine, liposomal
nanocurcumin, and PL pro nanocurcumin was found to
increase the level of hydroxyproline in the skin tissue
(vehicle-treated burned group vs. silver sulfadiazinetreated burned group, p=0.002; vehicle-treated burned
group vs. liposomal nanocurcumin-treated group,
p<0.001; vehicle-treated burned group vs. PL pro
nanocurcumin-treated group, p<0.001).
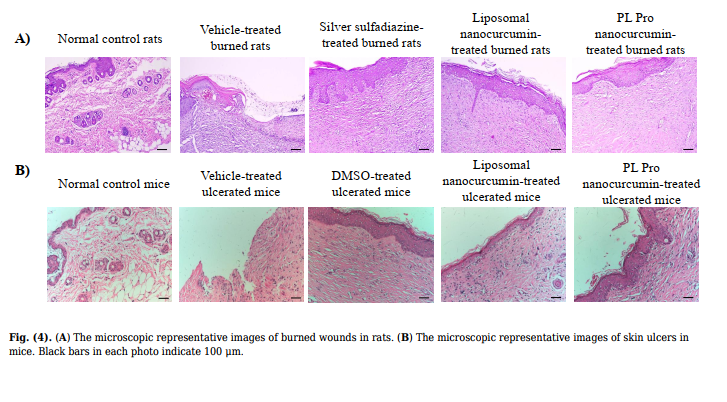
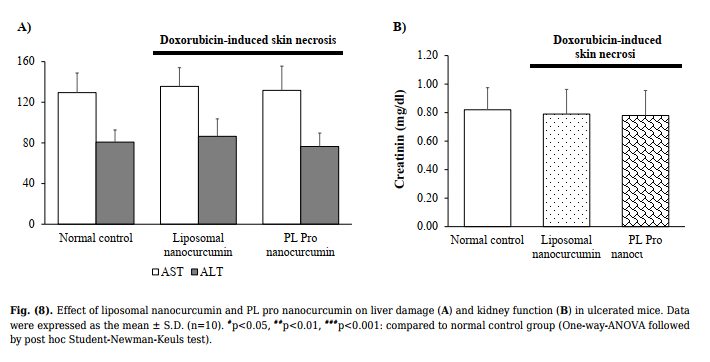
| 3.2.3. Histopathological Examination | cells between liposomal nanocurcumin and PL pro nanocurcumin-treated groups and normal control group |
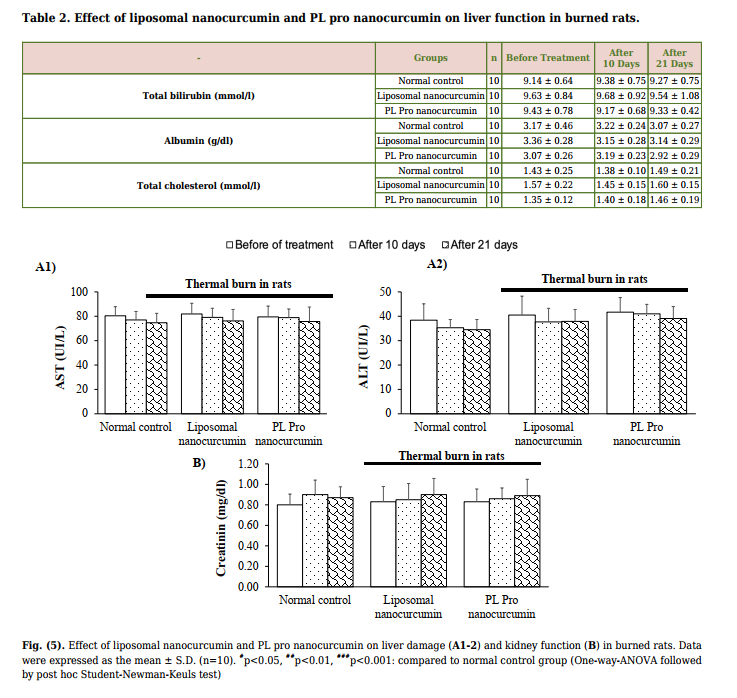
| The skin biopsy of the normal control rats demonstrated the proper and well-structured stratum epidermis with keratinization, clear basal lamina, skin dependent components in the dermis layer, loose connective tissue, and small blood vessels. Thus, in the normal control group, the skin structure of rats was found to be normal. In the vehicle-treated group, the rat skin tissue showed a large ulcerated area whilst the surface was covered with the necrotic substance erythrocyte fibrin, various inflammatory cells, neutrophils, and macrophages. On the 21st day, burn healing was better in silver sulfadiazine, liposomal nanocurcumin, and PL pro nanocurcumin-treated groups than in the vehicle-treated group (Fig. 4A-B). | (p>0.05). 3.3.2. Effect on Liver Damage, Liver Function, and Kidney Function Fig. (5A1-2 and 5B) demonstrate that liposomal nanocurcumin and PL pro nanocurcumin did not cause any statistical difference in AST, ALT levels, and creatinine levels when comparing the treated groups to the normal control group (p>0.05). The effect of liposomal nanocurcumin and PL pro nanocurcumin on the total bilirubin, albumin, and total cholesterol of the normal control group and treated groups are presented in Table 2. No statistical difference was observed between groups (p>0.05). In addition, there were no significant differences in histopathological examinations of livers and kidneys |
3.3. Evaluation of Systemic Toxicity of Topical
Administration of Liposomal Nanocurcumin and PL
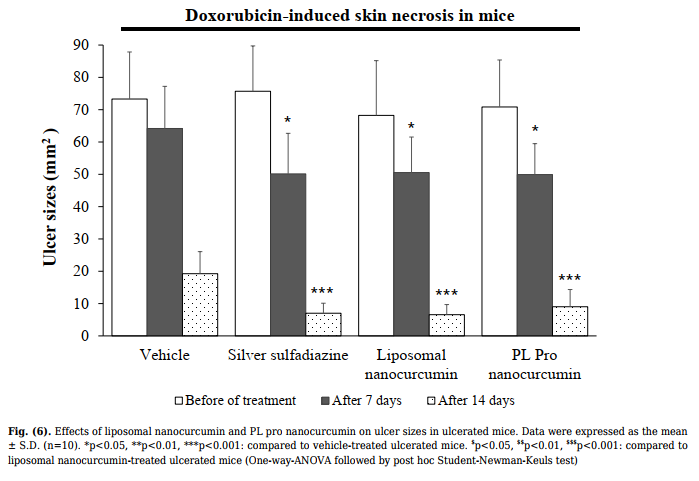
| Pro Nanocurcumin Creams in Burned Rats During the experimental period, there was an increase in body weight in each group of animals. No significant differences were found as compared to that of the control group. None of the animals in all treated groups showed any macroscopic or gross pathological changes when compared to the control group. No gross lesions or changes in size were observed when evaluating all experimental rats to a full gross necropsy, which examined the hearts, livers, lungs, kidneys, and abdominal cavities. | (Fig. S2A). 3.4. Healing Effects of Liposomal Nanocurcumin and PL Pro Nanocurcumin on Doxorubicin-induced Skin Ulcer in Mice 3.4.1. Effect on Ulcerated Area As shown in Fig. (6), no difference in the areas of skin ulcers was found between groups (p>0.05) for the time before treatment. After 7 and 21 days of administration, DMSO, liposomal nanocurcumin, and PL pro nanocurcumin significantly reduced the ulcer size |
| 3.3.1. Effect on Hematopoietic Function As mentioned in Table 1, there were no significant differences in red blood cell count, hematocrit, hemoglobin level, platelet count, and total white blood | compared to the vehicle-treated group (p<0.01). Additionally, there were no statistical differences in terms of reducing the skin lesions’ area between liposomal nanocurcumin and PL pro nanocurcumin (p>0.05). |
between liposomal nanocurcumin and PL pro
nanocurcumin-treated rats and the normal control group
Table 1. Effect of liposomal nanocurcumin and PL pro nanocurcumin on hematopoietic function in burned rats.
After 21 days of administration, the rate of wound
healing in vehicle-treated mice was 10%. The rate of wound
healing in the silver sulfadiazine- and the liposomal
nanocurcumin-treated group was 70% and 80%,
respectively (p=0.02 and p=0.005 compared to the vehicletreated group; Fisher’s Exact test). PL pro nanocurcumintreated mice had a wound healing rate of 60%. There was
no noticeably significant difference in the rate of wound
healing between the vehicle-treated group and the PL pro
nanocurcumin-treated group (p=0.057).
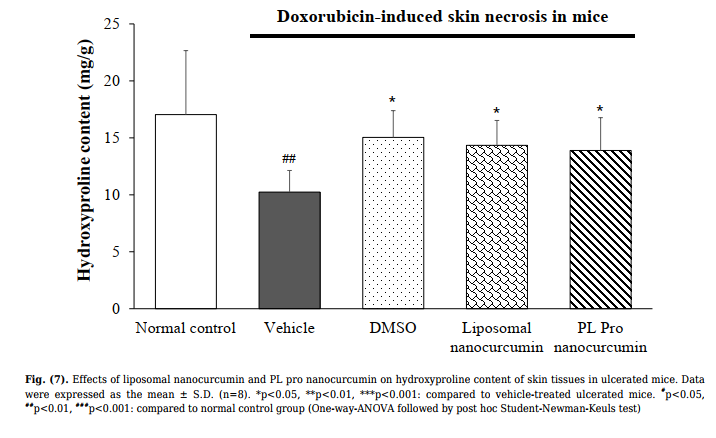
3.4.2. Effect on Hydroxyproline Content
The hydroxyproline content is presented in Fig. (7). The
hydroxyproline level in skin tissues of the vehicle-treated
group was significantly lower than the normal control group
(p<0.001). Compared with the vehicle-treated model group,
treatment of DMSO, liposomal nanocurcumin, and PL Pro
nanocurcumin significantly increased the hydroxyproline
content in the skin tissue. In addition, there were no
significant differences in the effects of liposomal
nanocurcumin and PL pro nanocurcumin on the
concentration of hydroxyproline in skin tissues (p>0.05).
3.4.3. Histopathological Examination
The skin biopsy of the normal control mice was normal,
with the proper stratum epidermis with keratinization,
clear basal lamina, skin-dependent components in the
dermis layer, loose connective tissue, and small blood
vessels. In the vehicle-treated ulcerated mice, the skin
tissue showed a large necrosis area, and the surface was
covered with necrotic substances, erythrocytes, fibrin,
many inflammatory cells, neutrophils, and macrophages.
On the 21st day, DMSO, liposomal nanocurcumin, and PL
pro nanocurcumin improved the histopathological
structure of skin tissues, which demonstrated the slight
growth of dermal papillae and epidermal ridges.
3.5. Evaluation of Systemic Toxicity of Topical
Administration of Liposomal Nanocurcumin and PL
Pro Nanocurcumin in Ulcerated Mice
During the experimental period, there was an increase
in body weight in each group of animals. No significant
differences were seen as compared to that of the control
group. None of the animals in all treated groups showed
any macroscopic or gross pathological changes when
compared to the control group. No gross lesions or changes
in size were observed when evaluating all experimental rats
to a full gross necropsy, which examined the hearts, livers,
lungs, kidneys, and abdominal cavities.
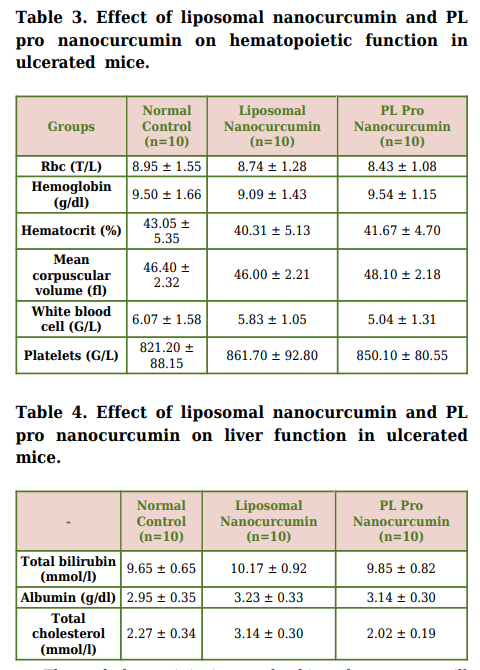
3.5.1. Effect on Hematopoietic Function
As mentioned in Table 3, there were no significant
differences in red blood cell count, hematocrit, hemoglobin
level, total white blood cell, and platelet count between
liposomal nanocurcumin and PL pro nanocurcumin-treated
groups and normal control group (p>0.05).
3.5.2. Effect on Liver Damage, Liver Function, and
Kidney Function
Fig. (8) demonstrates that liposomal nanocurcumin and
PL pro nanocurcumin did not cause any statistical
difference in AST, ALT level, and creatinine levels when
comparing the treated groups to the normal control group
(p>0.05). The effects of liposomal nanocurcumin and PL pro
nanocurcumin on the total bilirubin, albumin, and total
cholesterol of the normal control group and treated groups
are presented in Table 4. No statistical difference was
observed between groups (p>0.05).
Additionally, there were no significant differences in
histopathological examinations of livers and kidneys
between liposomal nanocurcumin and PL pro nanocurcumin-treated ulcerated and normal control mice (Fig.
S2B).
4. DISCUSSION
In this study, we not only evaluated the effect of
topical administration of liposomal nanocurcumin and PL
pro nanocurcumin on two different models of skin lesions,
which were induced, respectively, by heat and doxorubicin
but also detected any systemic toxicity of liposomal and PL
pro nanocurcumin via the ulcers in the experimental
animals. Our results showed that liposomal nanocurcumin
and PL pro nanocurcumin significantly reduced the size of
the wounded area, increased the hydroxyproline level in
skin tissues, and improved the histopathological structure
of skin tissues. Liposomal nanocurcumin showed better
effects than PL pro nanocurcumin on thermal burns in
rats. Additionally, topical administration of liposomal
nanocurcumin and PL pro nanocurcumin did not cause
systemic toxicity. Thus, liposomal nanocurcumin and PL
pro nanocurcumin have been shown to accelerate the
wound healing process without systemic toxicity in
experimental rat models.
Thermal burn injuries and skin ulcers are still
considered major health problems, resulting in physical
and psychological scars and disabilities [30]. Depending
on the lesion severity, wound healing is one of the most
complex processes, which involves several phases of
coagulation, inflammation, growth, re-epithelialization,
and remodeling [43, 44]. Research on burns has generated
sustained interest over the past few decades. However,
drugs for treating burns and skin ulcers are still limited
[36]. For the treatment of burns, silver sulfadiazine is
considered the gold standard in the topical treatment of
second-degree burns because of its antibacterial
properties. However, silver sulfadiazine is associated with
toxicity to keratinocytes and fibroblasts. So, this drug
delays the wound healing process and has some serious
cytotoxic effects on the host cells. Moreover, several
bacteria are resistant to silver sulfadiazine [36].
Additionally, for the treatment of skin ulcers, dimethyl
sulfoxide (DMSO) is a perennially proposed remedy since
it can easily infiltrate into the affected area and scavenge
free radicals [39]. However, currently, the pharmaceutical
form of DMSO is limited. Moreover, the accessibility of
DMSO in particular and other effective agents for skin
ulcers is still restricted. Considering this, there is an
emerging demand for a safer and more effective approach
to be applied in the treatment of wounds.
Curcumin possesses a powerful wound-healing effect
for the treatment of various skin disorders and damages
[5, 45-47]. In particular, curcumin attenuates the
inflammatory response and hastens wound healing by
increasing cellular proliferation and improving collagen
deposition in the wound tissues, as well as promoting
angiogenesis in chronic wounds [48-50]. Thus, curcumin
reinforces the healing of the affected tissue with different
levels of severity. Effect on inflammation, fibroblast
proliferation, granulation tissue formation, and collagen
deposition are mentioned mechanisms of the healing
potential of curcumin [51-53]. However, curcumin exhibits
several limitations in wound healing treatment, including
poor water solubility and physicochemical instability [21].
As a solution, nanoformulations should be applied in order
to deliver substance to the targets more accurately [20].
Specifically, liposomes with nano-sized phospholipid
bilayered vesicles were utilized for transport with a variety
of drugs, including wound healing agents. These are not
difficult to prepare and are highly biocompatible in nature.
This approach of nanoformulation has shown promising
results in the improvement of aqueous solubility of
curcumin and the development of a sustained and
prolonged drug-release system, thus enhancing wound
healing and closure [54]. In the present study, thermal
burn wounds in rats and doxorubicin-induced skin ulcers
in mice were used to evaluate the healing effect of topical
administration of liposomal nanocurcumin and PL pro
nanocurcumin. Liposomal nanocurcumin and PL pro
nanocurcumin significantly reduced the size of the
wounded area, increased the hydroxyproline content in
skin tissues, and improved the histopathological structure
of skin tissues. In addition to assessing the criteria for the
damaged area of skin ulcer, we also evaluated the
hydroxyproline level. According to the literature, collagen
plays a pivotal role in wound healing. Hydroxyproline is a
major component of the protein collagen, as it is a
principal component of connective tissues produced by
fibroblasts. It assists the wound in gaining tensile strength
during wound repair, hence serving as a structural
framework, strength, and milieu for the regenerating
tissue [55-68]. We determined collagen synthesis
indirectly by hydroxyproline level. Our results indicated
that liposomal nanocurcumin and PL pro nanocurcumin
increased the level of hydroxyproline in the skin tissue.
In this study, doxorubicin, a chemotherapeutic drug
belonging to the anthracyline group, was used as a skin
ulcerative agent. It is one of the most important drugs
causing skin necrosis and, ultimately, severe ulceration,
with the incidence of extravasation injury being 0.1% to
6.5% [41, 59, 60]. This agent could affect the replication
and translation process, as well as activate the gene that
is responsible for cellular apoptosis. Eventually, the ulcer
caused by doxorubicin injection was broad and deep,
indicating that the ulceration model triggered by
doxorubicin was adequately reliable in gauging the
efficiency of liposomal nanocurcumin and PL pro
nanocurcumin [61]. According to our previous study, the
development of skin necrosis reached its maximum size in
one week [62]. In order to alleviate the triggered skin
lesion, the newly formed radicals in the cytosol and
interstitial space should be eliminated by potent
antioxidants for clinical practice. Therefore, DMSO was
used as a positive control in this study. Our results
indicated that liposomal nanocurcumin and PL pro
nanocurcumin significantly reduced ulcer size. However,
there were no significant differences in the healing effect
between liposomal nanocurcumin and PL pro
nanocurcumin-treated groups. In addition, burns can be
defined as tissue lesions resulting from exposure to
thermal sources, such as flames, hot surfaces and liquids,
extreme cold, chemicals, radiation, or friction [32]. In this
study, the model of superficial second-degree burns on
rats was successfully induced. Interestingly, liposomal
nanocurcumin showed better effects than PL pro
nanocurcumin on thermal burns in rats. In addition,
liposomal nanocurcumin significantly reduced infection,
compared to the vehicle-treated group, by improving the
macroscopic and histopathological structure. Furthermore, it did not affect the number of white blood cells
when compared to the normal control group. These effects
underscore the efficacy of liposomal nanocurcumin in burn
treatment. Thus, liposomal nanocurcumin is a more potent
wound-healing agent.
We also evaluated the systemic toxicity after the
application of topical liposomal nanocurcumin and PL pro
nanocurcumin on thermal burns in rats and skin ulcers in
mice. Long-term topical application can also affect the
systemic effects, especially when applied to open wounds
[63]. Overall, the findings of this study indicated that
topical administration of liposomal nanocurcumin and PL
pro nanocurcumin caused no significant change in the
general status, haematological parameters, and renal and
hepatic functions. Additionally, they did not alter the
histology of the liver and kidneys in animals. In oral
administration, curcumin did not exert acute, subchronic,
chronic toxicity, or reproductive toxicity in animals [11,
12, 64]. To date, there have been no studies evaluating the
systemic toxicity of nanocurcumin in open wounds. Our
results indicated that liposomal nanocurcumin and PL pro
nanocurcumin did not cause systemic toxicity in burned
rats and ulcerated mice. Hence, these studies suggest the
beneficial effects of liposomal nanocurcumin and PL pro
nanocurcumin and the potential of these formulations to
be developed as a potent nontoxic agents for treating skin
disorders. Overall, liposomal nanocurcumin and PL pro
nanocurcumin are valuable in the near future for wound
healing, but additional studies are required to provide
scientists with a deeper understanding.
CONCLUSION
The current study demonstrated that the topical
application of liposomal nanocurcumin and PL pro
nanocurcumin creams exerted healing effects on burned
skin in rats and doxorubicin-induced skin ulcers in mice.
Furthermore, liposomal nanocurcumin and PL pro
nanocurcumin did not cause systemic toxicity in the
experimental model. Liposomal nanocurcumin showed
better effects than PL pro nanocurcumin on thermal burns
in rats.
AUTHORS’ CONTRIBUTIONS
It is hereby acknowledged that all authors have
accepted responsibility for the manuscript’s content and
consented to itssubmission. They have meticulously
reviewed all results and unanimously approved the final
version of the manuscript.
LIST OF ABBREVIATIONS
| DMSO | = Dimethyl Sulfoxide | |||
| AST ALT | = Aspartate Aminotransferase = Alanine Aminotransferase | |||
| ETHICS | APPROVAL | AND | CONSENT | TO |
PARTICIPATE
This study was approved by the Scientific Board
Committee of Hanoi Medical University, Vietnam (ref
number: IRB00003121).
HUMAN AND ANIMAL RIGHTS
All experimental protocols were in accordance with the
National Guidelines (reference number: 141/QD-K2DT).
This study adhered to internationally accepted standards
for animal research, following the 3Rs principle. The
ARRIVE guidelines were employed to report experiments
involving live animals and promote ethical research
practices.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is
available in the Zenodo Repository at https://zenodo.org/
records/11228442.
FUNDING
None.
CONFLICT OF INTEREST
The authors declared no conflict of interest, financial
or otherwise.
ACKNOWLEDGEMENTS
Declared none.
SUPPLEMENTARY MATERIALS
Supplementary material is available on the Publisher’s
website.
REFERENCES
[1] Esatbeyoglu T, Huebbe P, Ernst IMA, Chin D, Wagner AE,
Rimbach G. Curcumin-from molecule to biological function.
Angew Chem Int Ed 2012; 51(22): 5308-32.
http://dx.doi.org/10.1002/anie.201107724 PMID: 22566109
[2] Hewlings S, Kalman D. Curcumin: A review of its effects on
human health. Foods 2017; 6(10): 92.
http://dx.doi.org/10.3390/foods6100092 PMID: 29065496
[3] Mahady GB, Pendland SL, Yun G, Lu ZZ. Turmeric (Curcuma
longa) and curcumin inhibit the growth of Helicobacter pylori, a
group 1 carcinogen. Anticancer Res 2002; 22(6C): 4179-81.
PMID: 12553052
[4] Fernández-Bedmar Z, Alonso-Moraga A. In vivo and in vitro
evaluation for nutraceutical purposes of capsaicin, capsanthin,
lutein and four pepper varieties. Food Chem Toxicol 2016; 98(Pt
B): 89-99.
http://dx.doi.org/10.1016/j.fct.2016.10.011 PMID: 27746329
[5] Akbik D, Ghadiri M, Chrzanowski W, Rohanizadeh R. Curcumin as
a wound healing agent. Life Sci 2014; 116(1): 1-7.
http://dx.doi.org/10.1016/j.lfs.2014.08.016 PMID: 25200875
[6] Willenbacher E, Khan S, Mujica S, et al. Curcumin: new insights
into an ancient ingredient against cancer. Int J Mol Sci 2019;
20(8): 1808.
http://dx.doi.org/10.3390/ijms20081808 PMID: 31013694
[7] Silva AC, Santos PDF, Silva JTP, Leimann FV, Bracht L, Gonçalves
OH. Impact of curcumin nanoformulation on its antimicrobial
activity. Trends Food Sci Technol 2018; 72: 74-82.
http://dx.doi.org/10.1016/j.tifs.2017.12.004
[8] Jakubczyk K, Drużga A, Katarzyna J, Skonieczna-Żydecka K.
Antioxidant potential of curcumin—a meta-analysis of randomized
clinical trials. Antioxidants 2020; 9(11): 1092.
http://dx.doi.org/10.3390/antiox9111092 PMID: 33172016
[9] Peng Y, Ao M, Dong B, et al. Anti-Inflammatory Effects of
Curcumin in the Inflammatory Diseases: Status, Limitations and
Countermeasures. Drug Des Devel Ther 2021; 15: 4503-25.
http://dx.doi.org/10.2147/DDDT.S327378 PMID: 34754179
[10] Kumar B, Aggarwal R, Prakash U, Sahoo PK. Emerging
therapeutic potential of curcumin in the management of
dermatological diseases: an extensive review of drug and
pharmacological activities. Future J Pharmaceut Sci 2023; 9(1):
42.
http://dx.doi.org/10.1186/s43094-023-00493-1 PMID: 36620352
[11] Jantawong C, Priprem A, Intuyod K, et al. Curcumin-loaded
nanocomplexes: Acute and chronic toxicity studies in mice and
hamsters. Toxicol Rep 2021; 8: 1346-57.
http://dx.doi.org/10.1016/j.toxrep.2021.06.021 PMID: 34277359
[12] Murugan S, Solanki H, Purusothaman D, Bethapudi B, Ravalji M,
Mundkinajeddu D. Safety evaluation of standardized extract of
Curcuma longa (NR-INF-02): A 90-day subchronic oral toxicity
study in rats. BioMed Res Int 2021; 2021: 1-14.
http://dx.doi.org/10.1155/2021/6671853 PMID: 34337042
[13] Aggarwal ML, Chacko KM, Kuruvilla BT. Systematic and
comprehensive investigation of the toxicity of curcuminoidessential oil complex: A bioavailable turmeric formulation. Mol
Med Rep 2016; 13(1): 592-604.
http://dx.doi.org/10.3892/mmr.2015.4579 PMID: 26648561
[14] Damarla SR, Komma R, Bhatnagar U, Rajesh N, Mulla SMA. An
evaluation of the genotoxicity and subchronic oral toxicity of
synthetic curcumin. J Toxicol 2018; 2018: 1-27.
http://dx.doi.org/10.1155/2018/6872753 PMID: 30111997
[15] Ombredane AS, Silva VRP, Andrade LR, et al. In vivo efficacy and
toxicity of curcumin nanoparticles in breast cancer treatment: a
systematic review. Front Oncol 2021; 11: 612903.
http://dx.doi.org/10.3389/fonc.2021.612903 PMID: 33767985
[16] Tiwari R, Siddiqui MH, Mahmood T, et al. An exploratory analysis
on the toxicity & safety profile of Polyherbal combination of
curcumin, quercetin and rutin. Clinical Phytoscience 2020; 6(1):
82.
http://dx.doi.org/10.1186/s40816-020-00228-2
[17] Thangapazham RL, Sharma A, Maheshwari RK. Beneficial role of
curcumin in skin diseases. Adv Exp Med Biol 2007; 595: 343-57.
http://dx.doi.org/10.1007/978-0-387-46401-5_15 PMID: 17569219
[18] Tejada S, Manayi A, Daglia M, et al. Wound healing effects of
curcumin: A short review. Curr Pharm Biotechnol 2016; 17(11):
1002-7.
http://dx.doi.org/10.2174/1389201017666160721123109 PMID:
27640646
[19] Phan TT, See P, Lee ST, Chan SY. Protective effects of curcumin
against oxidative damage on skin cells in vitro: Its implication for
wound healing. J Trauma 2001; 51(5): 927-31.
http://dx.doi.org/10.1097/00005373-200111000-00017 PMID:
11706342
[20] Kumari A, Raina N, Wahi A, et al. Wound-healing effects of
curcumin and its nanoformulations: A comprehensive review.
Pharmaceutics 2022; 14(11): 2288.
http://dx.doi.org/10.3390/pharmaceutics14112288 PMID:
36365107
[21] Flora G, Gupta D, Tiwari A. Nanocurcumin: A promising
therapeutic advancement over native curcumin. Crit Rev Ther
Drug Carrier Syst 2013; 30(4): 331-68.
http://dx.doi.org/10.1615/CritRevTherDrugCarrierSyst.201300723
6 PMID: 23662605
[22] Liu S, Liu J, He L, et al. A comprehensive review on the benefits
and problems of curcumin with respect to human health.
Molecules 2022; 27(14): 4400.
http://dx.doi.org/10.3390/molecules27144400 PMID: 35889273
[23] Margiana R, Alawiyah K, Ima K. The disadvantages of curcumin
based on its phytochemical composition and anti-inflammatory
activity in peripheral nerve reeneration in sciatic nerve injury. Nat
Volatiles & Essent Oil 2021; 8(4): 8185-201.
[24] Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB.
Bioavailability of curcumin: Problems and promises. Mol Pharm
2007; 4(6): 807-18.
http://dx.doi.org/10.1021/mp700113r PMID: 17999464
[25] Gera M, Sharma N, Ghosh M, et al. Nanoformulations of
curcumin: An emerging paradigm for improved remedial
application. Oncotarget 2017; 8(39): 66680-98.
http://dx.doi.org/10.18632/oncotarget.19164 PMID: 29029547
[26] Yallapu MM, Nagesh PKB, Jaggi M, Chauhan SC. Therapeutic
applications of curcumin nanoformulations. AAPS J 2015; 17(6):
1341-56.
http://dx.doi.org/10.1208/s12248-015-9811-z PMID: 26335307
[27] Naksuriya O, Okonogi S, Schiffelers RM, Hennink WE. Curcumin
nanoformulations: A review of pharmaceutical properties and
preclinical studies and clinical data related to cancer treatment.
Biomaterials 2014; 35(10): 3365-83.
http://dx.doi.org/10.1016/j.biomaterials.2013.12.090 PMID:
24439402
[28] Hafez Ghoran S, Calcaterra A, Abbasi M, Taktaz F, Nieselt K,
Babaei E. Curcumin-based nanoformulations: A promising
adjuvant towards cancer treatment. Molecules 2022; 27(16):
5236.
http://dx.doi.org/10.3390/molecules27165236 PMID: 36014474
[29] Yallapu MM, Jaggi M, Chauhan SC. Curcumin nanoformulations: A
future nanomedicine for cancer. Drug Discov Today 2012; 17(1-2):
71-80.
http://dx.doi.org/10.1016/j.drudis.2011.09.009 PMID: 21959306
[30] Rowan MP, Cancio LC, Elster EA, et al. Burn wound healing and
treatment: Review and advancements. Crit Care 2015; 19(1): 243.
http://dx.doi.org/10.1186/s13054-015-0961-2 PMID: 26067660
[31] Yakupu A, Zhang J, Dong W, Song F, Dong J, Lu S. The
epidemiological characteristic and trends of burns globally. BMC
Public Health 2022; 22(1): 1596.
http://dx.doi.org/10.1186/s12889-022-13887-2 PMID: 35996116
[32] Jeschke MG, van Baar ME, Choudhry MA, Chung KK, Gibran NS,
Logsetty S. Burn injury. Nat Rev Dis Primers 2020; 6(1): 11.
http://dx.doi.org/10.1038/s41572-020-0145-5 PMID: 32054846
[33] Opriessnig E, Luze H, Smolle C, et al. Epidemiology of burn injury
and the ideal dressing in global burn care – Regional differences explored. Burns 2023; 49(1): 1-14.http://dx.doi.org/10.1016/j.burns.2022.06.018 PMID: 35843806
[34] Kim H, Shin S, Han D. Review of history of basic principles of
burn wound management. Medicina (Kaunas) 2022; 58(3): 400.
http://dx.doi.org/10.3390/medicina58030400 PMID: 35334576
[35] Wasiak J, Cleland H, Campbell F, Spinks A. Dressings for
superficial and partial thickness burns. Cochrane Libr 2013;
2013(3): CD002106.
http://dx.doi.org/10.1002/14651858.CD002106.pub4 PMID:
23543513
[36] Ibrahim NI, Mohamed IN, Mohamed N, Mohd Ramli ES, Shuid
AN. The effects of aqueous extract of Labisia Pumila (Blume)
Fern.-Vill. Var. Alata on wound contraction, hydroxyproline
content and histological assessments in superficial partial
thickness of second-degree burn model. Front Pharmacol 2022;
13: 968664.
http://dx.doi.org/10.3389/fphar.2022.968664 PMID: 36313379
[37] Heyneman A, Hoeksema H, Vandekerckhove D, Pirayesh A,
Monstrey S. The role of silver sulphadiazine in the conservative
treatment of partial thickness burn wounds: A systematic review.
Burns 2016; 42(7): 1377-86.
http://dx.doi.org/10.1016/j.burns.2016.03.029 PMID: 27126813
[38] Hashmi DL, Haith L Jr. The current state of topical burn
treatments: A review. Curr Trauma Rep 2019; 5(3): 160-8.
http://dx.doi.org/10.1007/s40719-019-00170-w
[39] Ludwid CU, Stoll HR, Obristl R, Obrecht JP. Prevention of
cytotoxic drug induced skin ulcers with dimethyl sulfoxide
(DMSO) and α-tocopherole. Eur J Cancer Clin Oncol 1987; 23(3):
327-9.
http://dx.doi.org/10.1016/0277-5379(87)90077-0 PMID: 3595692
[40] Durmus A, Han MC, Yaman I. Comperative evaluation of
collagenase and silver sulfadiazine on burned wound healing in
rats. F U Sag Bil Vet Derg 2009; 23(3): 135-9.
[41] Kesik V, Kurt B, Tunc T, et al. Melatonin ameliorates doxorubicininduced skin necrosis in rats. Ann Plast Surg 2010; 65(2): 250-3.
http://dx.doi.org/10.1097/SAP.0b013e3181bb4b4e PMID:
20585237
[42] Stegemann H, Stalder K. Determination of hydroxyproline. Clin
Chim Acta 1967; 18(2): 267-73.
http://dx.doi.org/10.1016/0009-8981(67)90167-2 PMID: 4864804
[43] Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res
2010; 89(3): 219-29.
http://dx.doi.org/10.1177/0022034509359125 PMID: 20139336
[44] Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound
healing: A cellular perspective. Physiol Rev 2019; 99(1): 665-706.
http://dx.doi.org/10.1152/physrev.00067.2017 PMID: 30475656
[45] Barchitta M, Maugeri A, Favara G, et al. Nutrition and wound
healing: An overview focusing on the beneficial effects of
curcumin. Int J Mol Sci 2019; 20(5): 1119.
http://dx.doi.org/10.3390/ijms20051119 PMID: 30841550
[46] Zhao Y, Dai C, Wang Z, et al. A novel curcumin-loaded composite
dressing facilitates wound healing due to its natural antioxidant
effect. Drug Des Devel Ther 2019; 13(Sep): 3269-80.
http://dx.doi.org/10.2147/DDDT.S219224 PMID: 31571829
[47] Yen YH, Pu CM, Liu CW, et al. Curcumin accelerates cutaneous
wound healing via multiple biological actions: The involvement of
TNF‐α, MMP‐9, α‐SMA, and collagen. Int Wound J 2018; 15(4):
605-17.
http://dx.doi.org/10.1111/iwj.12904 PMID: 29659146
[48] Panchatcharam M, Miriyala S, Gayathri VS, Suguna L. Curcumin
improves wound healing by modulating collagen and decreasing
reactive oxygen species. Mol Cell Biochem 2006; 290(1-2): 87-96.
http://dx.doi.org/10.1007/s11010-006-9170-2 PMID: 16770527
[49] Emiroglu G, Ozergin Coskun Z, Kalkan Y. The effects of curcumin
on wound healing in a rat model of nasal mucosal trauma. Evid
Based Complement Alternat Med 2017; 2027: 9452392.
http://dx.doi.org/10.1155/2017/9452392
[50] Heydari P, Zargar Kharazi A, Asgary S, Parham S. Comparing the
wound healing effect of a controlled release wound dressing
containing curcumin/ciprofloxacin and simvastatin/ciprofloxacin
in a rat model: A preclinical study. J Biomed Mater Res A 2022;
110(2): 341-52.
http://dx.doi.org/10.1002/jbm.a.37292 PMID: 34378857
[51] Adeliana , Usman AN, Ahmad M, Arifuddin S, Yulianty R,
Prihantono . Effectiveness of turmeric (Curcuma Longa Linn) Gel
Extract (GE) on wound healing: Pre-clinical test. Gac Sanit 2021;
35 (Suppl. 2): S196-8.
http://dx.doi.org/10.1016/j.gaceta.2021.07.014 PMID: 34929810
[52] Yahya EB, Setiawan A, Wibowo MD, Danardono E. The effect of
topical curcumin extract on fibroblast count and collagen density
as an indicator on accelerating clean wound healing process: A
study on Wistar rats. Sys Rev Pharm 2020; 11(11): 567-70.
[53] Heng M. Topical curcumin: A review of mechanisms and uses in
dermatology. Int J Dermatol Clin Res 2017; 1: 20.
http://dx.doi.org/10.17352/2455-8605.000020
[54] Choudhary V, Shivakumar H, Ojha H. Curcumin-loaded liposomes
for wound healing: Preparation, optimization, in-vivo skin
permeation and bioevaluation. J Drug Deliv Sci Technol 2019; 49:
683-91.
http://dx.doi.org/10.1016/j.jddst.2018.12.008
[55] Martin P. Wound healing–aiming for perfect skin regeneration.
Science 1997; 276(5309): 75-81.
http://dx.doi.org/10.1126/science.276.5309.75 PMID: 9082989
[56] Li P, Wu G. Roles of dietary glycine, proline, and hydroxyproline
in collagen synthesis and animal growth. Amino Acids 2018;
50(1): 29-38.
http://dx.doi.org/10.1007/s00726-017-2490-6 PMID: 28929384
[57] Kumar Srivastava A, Khare P, Kumar Nagar H, Raghuwanshi N,
Srivastava R. Hydroxyproline: A potential biochemical marker and
its role in the pathogenesis of different diseases. Curr Protein
Pept Sci 2016; 17(6): 596-602.
http://dx.doi.org/10.2174/1389203717666151201192247 PMID:
26916157
[58] Albaugh VL, Mukherjee K, Barbul A. Proline precursors and
collagen synthesis: Biochemical challenges of nutrient
supplementation and wound healing. J Nutr 2017; 147(11):
2011-7.
http://dx.doi.org/10.3945/jn.117.256404 PMID: 28978679
[59] Ener RA, Meglathery SB, Styler M. Extravasation of systemic
hemato-oncological therapies. Ann Oncol 2004; 15(6): 858-62.
http://dx.doi.org/10.1093/annonc/mdh214 PMID: 15151940
[60] Razavi-Azarkhiavi K, Iranshahy M, Sahebkar A, Shirani K, Karimi
G. The protective role of phenolic compounds against doxorubicininduced cardiotoxicity: A comprehensive review. Nutr Cancer
2016; 68(6): 892-917.
http://dx.doi.org/10.1080/01635581.2016.1187280 PMID:
27341037
[61] Box VGS. The intercalation of DNA double helices with
doxorubicin and nagalomycin. J Mol Graph Model 2007; 26(1):
14-9.
http://dx.doi.org/10.1016/j.jmgm.2006.09.005 PMID: 17046298
[62] Anh PTV, Huy VQ, Loan NTT. The effects of Kem con ong and Kem
tri bong creams on doxorubicin-induced skin ulcer in rats. J Med
Res 2023; 166: 11.
http://dx.doi.org/10.52852/tcncyh.v166i5E12.1523
[63] Alikhan FS, Maibach H. Topical absorption and systemic toxicity.
Cutan Ocul Toxicol 2011; 30(3): 175-86.
[64] Soleimani V, Sahebkar A, Hosseinzadeh H. Turmeric ( Curcuma
longa ) and its major constituent (curcumin) as nontoxic and safe
substances: Review. Phytother Res 2018; 32(6): 985-95.
http://dx.doi.org/10.1002/ptr.6054 PMID: 29480523